Copper Oxide and Sulfuric Acid Formula
The data requirements for pesticide registration include the submission of information on the composition of the pesticide product. Sulfuric Acid Boiling Point.

Pdf Copper Dissolution In Concentrated Sulfuric Acid
The compound can appear either yellow or red depending on the size of the particles.

. Even materials considered pure elements often develop an oxide coating. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Vinegar is considered a type of weak acid.
Their most familiar applications are in clothing food packaging and plastic water and carbonated soft drinks. It is approximately 5-20 acetic acid in water. A solution contains the following ions.
Calculate the number of milliequivalents of positive and negative charge respectively. So there are actually two main chemical formulas involved. Nitric acid is the inorganic compound with the formula HNO₃.
Etc per mole of substance. Polyesters are polymers formed from a dicarboxylic acid and a diol. The structural formula for acetic acid is CH 3 COOH.
Although it has an extremely low pH value the acetic acid doesnt completely dissociate in water. It is soluble in water and releases heat on contact. Sulfuric acid is a very strong acid.
Sulfuric acid reacts with most metals particularly when diluted with water to form flammable hydrogen gas which may create an explosion hazard. Pictured above is a 100ml bottle of concentrated nitric acid 70 68 - 70 ACS Reagent Grade which can be purchased online here. Sulfuric Acid Structure H2SO4.
Most of the Earths crust consists of oxides. On top of that this acid is corrosive to metals and most other organic matters like tissue wood etc. Polyesters are extremely important polymers.
EPA intends to update this list periodically. It is a colorless to slightly yellow odorless and viscous liquid soluble in water and alcohol used in many applications. It has a chemical formula H 2 SO 4.
Sulfuric Acid figures among the most important chemicals globally in 2012 about 230 million. For example for H2SO4 each mole of sulfuric acid yields two moles of protons so K 2 and the EW ½ MW. With oxygen in the oxidation state of 2.
H ydrogen sulfate ions further ionise in very dilute solutions to give sulphate ionsSO 4 2-. It is a highly corrosive mineral acid. Commercially available nitric acid is an azeotrope with water at a concentration of 68 HNO₃.
Registrants and applicants completing the Confidential Statement of Formula CSF Form EPA Form 8570-4 will no longer need to list the commodity inert ingredient suppliers. It is one of the principal oxides of copper the other being or copperII oxide or cupric oxide CuO. The molecular formula for water is H 2 O.
CopperI oxide is found as the reddish. It forms aqua regia when mixed with hydrochloric acid. This acid is colourless with a pungent smell.
Oxide itself is the dianion of oxygen an O 2 molecular ion. CopperI oxide or cuprous oxide is the inorganic compound with the formula Cu 2 O. On contact with such organic substances sulphuric acid instantly dehydrates them causing char to form.
In aqueous solutions it ionizes completely to form hydronium ions H 3 O and hydrogen sulfate ions HSO 4. Sulfuric Acid CAS Number. They have many uses depending on how they have been produced and the resulting orientation of the polymer chains.
Normality K x Molarity----- Sample Exercises. Properties of Sulfuric Acid H2SO4. This red-coloured solid is a component of some antifouling paints.
Sulfuric Acid Chemical Properties Hazards And Uses. Approximately 50 of the produced sulfuric acid is used in the fertilizer industry. H2SO4 is an inorganic compound in the acid class.
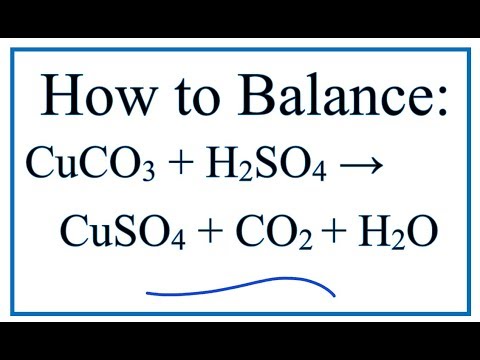
How To Balance Cuco3 H2so4 Cuso4 Co2 H2o Youtube

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube
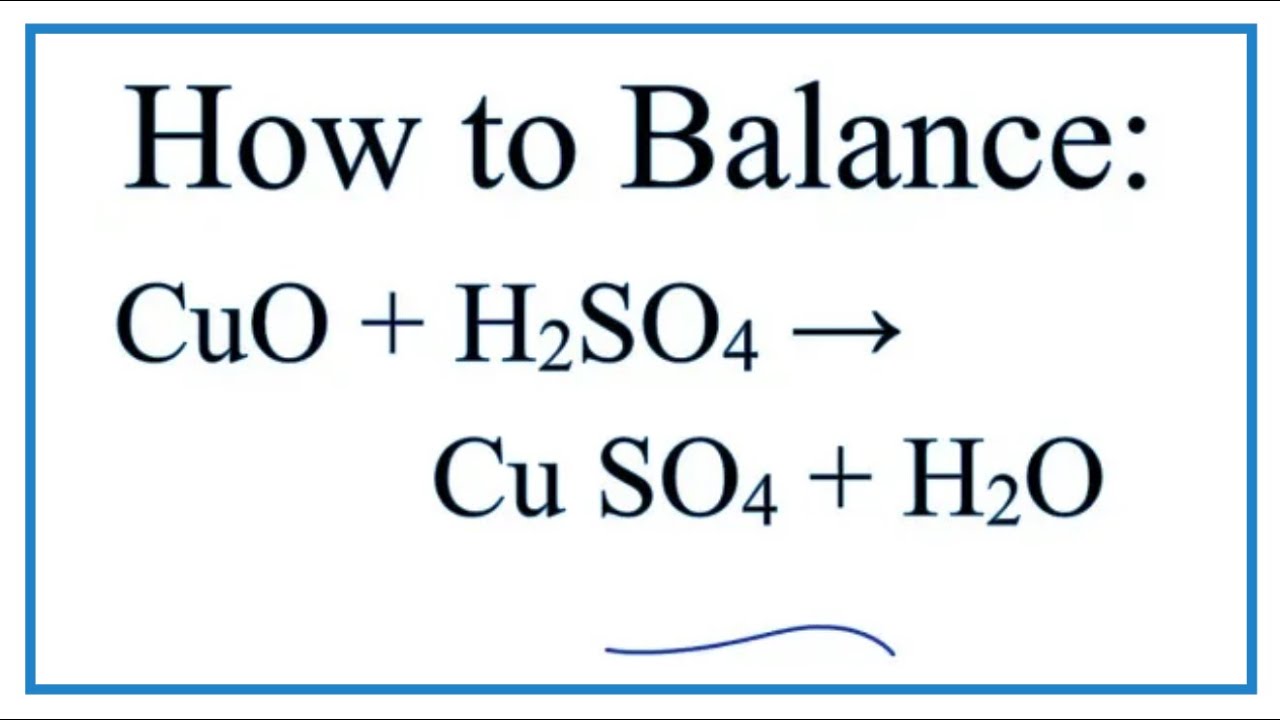
No comments for "Copper Oxide and Sulfuric Acid Formula"
Post a Comment